GrabCAD
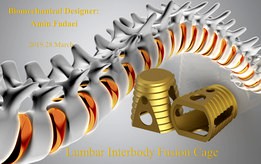
lumbar interbody fusion cage
by GrabCAD
Last crawled date: 1 year, 11 months ago
The Infuse® Bone Graft/LT-Cage® Lumbar Tapered Fusion Device is designed to aid in the treatment of degenerative disc disease (DDD) in skeletally mature patients at one level from L2-S1. It consists of two parts – the LT-Cage Lumbar Tapered Fusion Device and a bone graft alternative* (Infuse Bone Graft). The LT-Cage Lumbar Tapered Fusion Device component is intended to restore the degenerated disc space to its original height. Two implants are placed side by side.
The Infuse Bone Graft component is used to fill the LT-Cage Lumbar Tapered Fusion Device. The Infuse Bone Graft consists of two parts – a solution containing recombinant human bone morphogenetic protein-2 (rhBMP-2) and an absorbable collagen sponge (ACS). The protein is a manufactured (genetically engineered) version of a natural protein normally found in small quantities in the body.
The purpose of the protein is to stimulate bone formation. During surgery, the protein solution is soaked into the ACS. The ACS acts as a scaffold for the formation of new bone that the protein stimulates. The ACS is a sponge manufactured from Type I collagen, and is designed to resorb over time.
The Infuse Bone Graft component is used to fill the LT-Cage Lumbar Tapered Fusion Device. The Infuse Bone Graft consists of two parts – a solution containing recombinant human bone morphogenetic protein-2 (rhBMP-2) and an absorbable collagen sponge (ACS). The protein is a manufactured (genetically engineered) version of a natural protein normally found in small quantities in the body.
The purpose of the protein is to stimulate bone formation. During surgery, the protein solution is soaked into the ACS. The ACS acts as a scaffold for the formation of new bone that the protein stimulates. The ACS is a sponge manufactured from Type I collagen, and is designed to resorb over time.
Similar models
grabcad
free

Modular Bioreactor Design
...f a group project. the system would be used to grow bone grafts using indirect perfusion and mechanical stimulation of scaffolds.
thingiverse
free

Sponge Drying Rack by H_Alex
... the two parts for easy cleaning.
the parts are designed in inches, so increase their size by 25.4x to convert to millimeters.
3d_export
$5

vertebral implants lumbar cage m4 thread real operation
...ter the operation. lifelong fusion of the vertebrae occurs within 4-6 months, when bone fusion of the vertebrae occurs – fusion.
grabcad
free

spine implant cage
...rpose of using cages is often to restore lost disc height resulting from a collapsed disc and to relieve pressure on nerve roots.
3dwarehouse
free

Tapered (2-Sided) Dining Table Leg (29' x 2-3/4')
...ood choice when simplicity is the keynote of your project. it is also an excellent selection for making a contemporary statement.
grabcad
free

injection device into the bone tissue.
...e bone screw into the bone. the channel allows a bone screw to infuse antibiotic or other liquid substances.used in bone disease.
3dwarehouse
free

Tapered (2-Sided) Kitchen Island Leg (34-1/2' x 3-1/2')
...g works well with any type of countertop from granite to stainless steel, the simplicity of the leg does not overwhelm the space.
cg_trader
$15

Vertebra bone model Cervical vertebra thoracic vertebra lumbar
...a bone model cervical vertebra thoracic vertebra lumbar vertebra sacrum coccyx human bone medical model intervertebral disc spine
cg_trader
$10

Lumbar Spine - male
...rtebrae experience more body weight than other parts of the spinal column and are characterized by their large vertebral bodies.
grabcad
free

Dakar Rally Roll Cage - 2021
...dakar rally roll cage - 2021
grabcad
this cage has been manufactured from fusion 360 this year.
Interbody
3d_export
$5

vertebral implants lumbar cage m4 thread real operation
...and transpedicular screws. this method is called tlif-transforaminal lumbar interbody fusion. during this intervention, the entire disc is removed...
grabcad
free

spine implant cage
...these devices are sometimes called 'interbody cages.' the word 'interbody#39; refers to where these cages are used (i.e. the...
grabcad
free

ARION expandable bladed cervical peek cage
...of implants that has received the european approval. the interbody cages, designed to promote spinal fusion and healing, were...
Lumbar
turbosquid
$30

Spine Lumbar Vertebra
...y free 3d model spine. lumbar vertebra. for download as blend on turbosquid: 3d models for games, architecture, videos. (1568745)
3d_export
$24

Lumbar Vertebrae 3D Model
...bar medical skeletal skeleton vertebrae realistic detailed textures materials
lumbar vertebrae 3d model guidovrola 47146 3dexport
turbosquid
$39

Lumbar Spinal Column Bone
...d model lumbar spinal column bone for download as max and fbx on turbosquid: 3d models for games, architecture, videos. (1684226)
cg_studio
$79

Human Lumbar Vertebrae3d model
... .xsi .obj .max .ma .lxo .lwo - human lumbar vertebrae 3d model, royalty free license available, instant download after purchase.
3d_export
$79

Human Lumbar Vertebrae 3D Model
...anatomy 3ds lwo
human lumbar vertebrae 3d model download .c4d .max .obj .fbx .ma .lwo .3ds .3dm .stl digitallab3d 106348 3dexport
3d_export
$25

Lumbar Vertebrae and Spinal Cord 3D Model
...leton spine biology skeletal backbone bones characters internal
lumbar vertebrae and spinal cord 3d model vertexus 52502 3dexport
turbosquid
$23

Lumbar Gym Equipment Training Back Machine
...pment training back machine for download as obj, c4d, and fbx on turbosquid: 3d models for games, architecture, videos. (1376294)
turbosquid
$6

Lumbar Spine Osteoarthritis - L2 vertebrae - male 3D model
... available on turbo squid, the world's leading provider of digital 3d models for visualization, films, television, and games.
3d_export
$5

vertebral implants lumbar cage m4 thread real operation
...ter the operation. lifelong fusion of the vertebrae occurs within 4-6 months, when bone fusion of the vertebrae occurs – fusion.
3ddd
$1

Silla Ejecutiva
..., кресло silla ejecutiva con respaldo ergonomico y soporte lumbar base de estrella de 5 puntas y rodajas tipo...
Cage
3d_export
$5

cage
...cage
3dexport
cage
archibase_planet
free

Cage
...cage
archibase planet
cage bird cage birdcage
cage 2 - 3d model (*.gsm+*.3ds) for interior 3d visualization.
archibase_planet
free

Cage
...cage
archibase planet
cage bird cage birdcage
cage 1 - 3d model (*.gsm+*.3ds) for interior 3d visualization.
archibase_planet
free

Cage
...cage
archibase planet
cage bird cage birdcage
cage 3 - 3d model (*.gsm+*.3ds) for interior 3d visualization.
archibase_planet
free

Cage
...cage
archibase planet
bird's cage cage
cage n020909 - 3d model (*.gsm+*.3ds) for interior 3d visualization.
archibase_planet
free

Cage
...cage
archibase planet
cage
cage monte- 3d model for interior 3d visualization.
3d_export
$7

cage
...cage
3dexport
3d cage model
archibase_planet
free

Cage
...cage
archibase planet
cage
cage n270211 - 3d model (*.gsm+*.3ds) for interior 3d visualization.
turbosquid
$5

Cage
...ge
turbosquid
royalty free 3d model cage for download as dwg on turbosquid: 3d models for games, architecture, videos. (1224275)
turbosquid
$4

cage
...squid
royalty free 3d model cage for download as max and fbx on turbosquid: 3d models for games, architecture, videos. (1368360)
Fusion
3ddd
free

BoConcept Fusion
...boconcept fusion
3ddd
boconcept , fusion
boconcept fusion
3ddd
$1

Ford Fusion
...ford fusion
3ddd
ford
ford fusion
3ddd
$1

HANSA Fusion
...hansa fusion
3ddd
духовой шкаф , hansa
встраиваемый духовой шкаф hansa fusion
design_connected
$11

1520 Fusion
...1520 fusion
designconnected
dränert 1520 fusion computer generated 3d model. designed by aisslinger, werner.
3ddd
free

Диван BoConcept Fusion
...03]
артикул: 4990059fu032103
ссылка на продукт:
http://www.boconcept.com/ru-ru/furniture/living/sofas/fusion/10347/диван-fusion
3ddd
$1

Matthew Robinson / Fusion
... журнальный
solid wood tables matthew robinson / fusion
http://www.matthewrobinsonfurniture.co.uk/#!fusion
3ddd
$1

Best. Fusion
...а пристенная best fusion.
5930 полигонов.
wall mounted range hood best fusion.
5930 polygons.
file: max 2010, 2012; fbx; obj.
3ddd
free

Туалетный столик Fusion
...столик fusion
3ddd
трюмо , fusion
туалетный столик fusion
арт.108dwk
размеры: 120х47х76
design_connected
$13

Fusion Chair
...gnconnected
photo-realistic 3d models of the fusion chair from boconcept for 3d architectural and interior design presentations.
3ddd
$1

Кресло fusion
...ах ткани и кожи. на фотографии, фетр светло-серого цвета 2121/шпон дуба. в74xш97xг78см. [стулья - l042]
артикул: 4060058l0422121
