Thingiverse
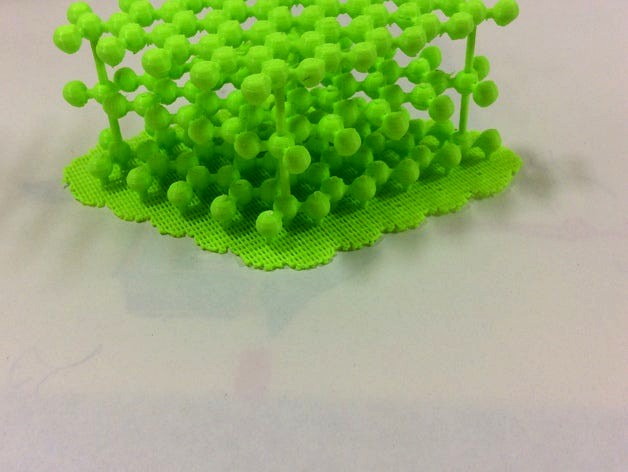
Graphite Atomic Structure by otomamay
by Thingiverse
Last crawled date: 4 years, 8 months ago
This is an atomic structure of graphite. Graphite is a layered material made of carbon atoms, and a pencil lead is made of graphite.
By printing this material, you can understand the characteristics of graphite structure such as:
a single layer has honeycomb structure which is due to the sp2 bondings between carbon atoms,
interlayer distance is much larger than intralayer distance. This is because the interlayer coupling is not covalent bonding but the van der Waals interaction (or intermolecular force),
so, you can feel a van der Waals world by this material.
The stl file is made by Blender.
By printing this material, you can understand the characteristics of graphite structure such as:
a single layer has honeycomb structure which is due to the sp2 bondings between carbon atoms,
interlayer distance is much larger than intralayer distance. This is because the interlayer coupling is not covalent bonding but the van der Waals interaction (or intermolecular force),
so, you can feel a van der Waals world by this material.
The stl file is made by Blender.
