Thingiverse
CYP1A2 (Cytochrome P450 1A2) by sokamarintchak
by Thingiverse
Last crawled date: 3 years ago
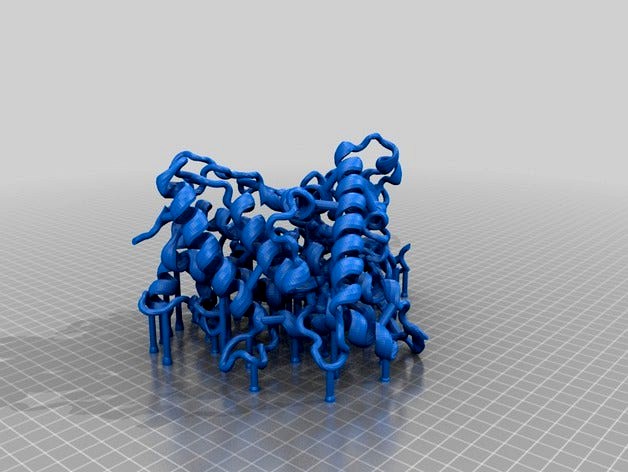
Imaged is my first printout of a PDB 2HI4 molecule cross-section (Cytochrome P450 1A2, or CYP1A2 for short, complex with alpha-napthoflavone ligand). A cross-section rendition was chosen to facilitate identification of the active site, heme cofactor, ligand, and highlighted amino acids.
What is CYP1A2 and why is it important?
CYP1A2 is a member of the cytochrome P450 enzyme family. It is involved in the metabolism of many different drugs, such as the blood thinner Clopidogrel (trade name: Plavix) and everyone's favorite, caffeine! Non-synonymous mutations (also known as missense mutations) occur as a result of small nucleotide polymorphisms (SNPs). Missense SNPs within the gene encoding for CYP1A2 can modulate the protein's.
Missense SNPs in CYP1A2 are found in different frequencies across populations and can be used as bio-markers for physicians. DNA sequencing technology can allow physicians to know a patient's genetic makeup at many different genes. By knowing a patient's bio-markers for gene encoding for the CYP1A2 protein, physicians can determine drug prescription dosages that are just right for that individual based on what their genetic bio-markers say. This is the basis of pharmacogenomics.
What's special about this model?:
I have highlighted in this model a few amino acids of interest. These amino acids are rendered in CPK format:
1). rs28399418 marks a missense SNP at the 314th amino acid (Ile314Val). This amino acid residue is located within the active site. Furthermore, the isoleucine residue is highly conserved between many mammals. This suggests that Ile314 plays an important role in CYP1A2's activity. This residue is included in the model and is located near the heme cofactor. It is the amino acid not bound to the heme cofactor.
2). rs28399424 marks a missense SNP at the 431st amino acid (Arg431Trp). This amino acid residue is located outside of the active site. The BLOSUM substitution score for this substitution is -2 and the arginine residue is also highly conserved between mammals. This suggests that Arg431 most likely plays a key role in maintaining proper structure of the protein. A tryptophan substitution most likely will disrupt the proteins structure at this site given that arginine (positively-charged and hydrophillic) and tryptophan (bulky and hydrophobic) are very biochemically different. In the model, Arg431 is the only amino acid highlighted that lies outside of the active site in the periphery.
3). The final highlighted amino acid is the 314th amino acid which is a cysteine residue. Cys314 closely interacts with the heme cofactor. It is likely that mutation from this cysteine to any other amino acid would greatly alter the activity of CYP1A2. In the model, this is the only amino acid that touches the heme cofactor.
It should be noted that mutations of the highlighted residues have not been studied experimentally for clinical relevance so no firm conclusions can be made. The conclusions made above are conjectures based off of fundamental knowledge of amino acids, protein structure, and conservation evolution. This model was made as a teaching tool for providing visual insight on missense SNP mutations and their applications into the evolving field of pharmacogenomics.
References:
1) Henikoff, S., & Henikoff, J. G. (1992). Amino acid substitution matrices from protein blocks. Proceedings of the National Academy of Sciences of the United States of America, 89(22), 10915–10919. http://doi.org/10.1073/pnas.89.22.10915
2) UCSC Genome Browser: Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002 Jun;12(6):996-1006. http://genome.ucsc.edu/
3) PDB ID: 2HI4
Sansen, S., Yano, J. K., Reynald, R. L., Schoch, G. A., Griffin, K. J., Stout, C. D., & Johnson, E. F. (2007). Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. Journal of Biological Chemistry, 282(19), 14348–14355. http://doi.org/10.1074/jbc.M611692200
What is CYP1A2 and why is it important?
CYP1A2 is a member of the cytochrome P450 enzyme family. It is involved in the metabolism of many different drugs, such as the blood thinner Clopidogrel (trade name: Plavix) and everyone's favorite, caffeine! Non-synonymous mutations (also known as missense mutations) occur as a result of small nucleotide polymorphisms (SNPs). Missense SNPs within the gene encoding for CYP1A2 can modulate the protein's.
Missense SNPs in CYP1A2 are found in different frequencies across populations and can be used as bio-markers for physicians. DNA sequencing technology can allow physicians to know a patient's genetic makeup at many different genes. By knowing a patient's bio-markers for gene encoding for the CYP1A2 protein, physicians can determine drug prescription dosages that are just right for that individual based on what their genetic bio-markers say. This is the basis of pharmacogenomics.
What's special about this model?:
I have highlighted in this model a few amino acids of interest. These amino acids are rendered in CPK format:
1). rs28399418 marks a missense SNP at the 314th amino acid (Ile314Val). This amino acid residue is located within the active site. Furthermore, the isoleucine residue is highly conserved between many mammals. This suggests that Ile314 plays an important role in CYP1A2's activity. This residue is included in the model and is located near the heme cofactor. It is the amino acid not bound to the heme cofactor.
2). rs28399424 marks a missense SNP at the 431st amino acid (Arg431Trp). This amino acid residue is located outside of the active site. The BLOSUM substitution score for this substitution is -2 and the arginine residue is also highly conserved between mammals. This suggests that Arg431 most likely plays a key role in maintaining proper structure of the protein. A tryptophan substitution most likely will disrupt the proteins structure at this site given that arginine (positively-charged and hydrophillic) and tryptophan (bulky and hydrophobic) are very biochemically different. In the model, Arg431 is the only amino acid highlighted that lies outside of the active site in the periphery.
3). The final highlighted amino acid is the 314th amino acid which is a cysteine residue. Cys314 closely interacts with the heme cofactor. It is likely that mutation from this cysteine to any other amino acid would greatly alter the activity of CYP1A2. In the model, this is the only amino acid that touches the heme cofactor.
It should be noted that mutations of the highlighted residues have not been studied experimentally for clinical relevance so no firm conclusions can be made. The conclusions made above are conjectures based off of fundamental knowledge of amino acids, protein structure, and conservation evolution. This model was made as a teaching tool for providing visual insight on missense SNP mutations and their applications into the evolving field of pharmacogenomics.
References:
1) Henikoff, S., & Henikoff, J. G. (1992). Amino acid substitution matrices from protein blocks. Proceedings of the National Academy of Sciences of the United States of America, 89(22), 10915–10919. http://doi.org/10.1073/pnas.89.22.10915
2) UCSC Genome Browser: Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002 Jun;12(6):996-1006. http://genome.ucsc.edu/
3) PDB ID: 2HI4
Sansen, S., Yano, J. K., Reynald, R. L., Schoch, G. A., Griffin, K. J., Stout, C. D., & Johnson, E. F. (2007). Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. Journal of Biological Chemistry, 282(19), 14348–14355. http://doi.org/10.1074/jbc.M611692200
Similar models
thingiverse
free

Cytochrome c by aarono
...ckbone is represented in "cartoon" form. the heme group and residues ligating the heme iron are represented as spheres.
thingiverse
free

FeMo-cofactor from Nitrogenase by MoleculeMaker
...inting of the model, and are meant to break off easily once the model is completed. this includes a histidine and cysteine ligand
thingiverse
free

Crambin Protein by aarono
... entry 1crn:
http://www.rcsb.org/pdb/explore.do?structureid=1crn
model created using vmd:http://www.ks.uiuc.edu/research/vmd/
cg_trader
$55

Collection of 21 alpha amino acids L- stereoisomers
...s. this collection contains the standard set of such compounds from which almost all proteins are made. this collection contains:
thingiverse
free

Protein Structure for PDB 3U8V by MoleculeMaker
.... this is a natural protein, found in the organism nitrosomonas europaea, which is commonly found in wastewater treatment plants.
thingiverse
free

Amino Acids Expansion Set by Eagleknot
...lable on my teacher pay teacher site, https://www.teacherspayteachers.com/product/3d-printable-dna-rna-manipulative-lab-1-3452084
thingiverse
free

Retinol binding protein (RBP) from PDB file 1RBP by TommieHata
...played inside the barrel. the rest of the protein is in "ribbon" view while the retinol is in "spheres" view.
thingiverse
free

Translation Modeling Activity by rforster2019
...ether depends on the sequence of bases in the mrna. proteins can consist of as few as 100 or as many as thousands of amino acids.
thingiverse
free

Dimer of Sickle Cell Hemoglobin Proteins by contentbydesign
...utation. look for the deoxy and oxy forms of hemoglobin to complete the set.
this model was created from the 2hbs pdb protein.
cg_trader
free

Deoxyribonucleic acid DNA
...chromosome molecule genetics deoxyribonucleic biology medical genetic genes gene organism chromosomes genome science dna molecule
Cytochrome
turbosquid
$20

cytochrome
... available on turbo squid, the world's leading provider of digital 3d models for visualization, films, television, and games.
thingiverse
free

Cytochrome c by aarono
...ckbone is represented in "cartoon" form. the heme group and residues ligating the heme iron are represented as spheres.
thingiverse
free

Cytochrome P450 3A4 by hectorsm
...db id: 1w0e
obtained from:https://www.rcsb.org/structure/1w0e
processed with:
blender (v.2.92.0)
pymol (v.2.4.1)
slic3r (v.1.3.0)
thingiverse
free

Cytochrome C (Human) (PDB 1J3S) by MoleculeMaker
... of the fluidity of the protein backbone and structure. it is not as sturdy as some of my other models, but turned out very nice.
thingiverse
free
Ferritin - The Cellular Iron Storage by EnriqueBumbury
...oxygen to diffuse throughout the muscle cells, and the cytochrome, which supply the body with its energy currency. other...
grabcad
free

정품졸피뎀가격,카톡【AKR331】라인【SPR331】졸피뎀구매처,졸피뎀부작용
...사용과 주의 깊은 모니터링이 필요하다"고 조언했다. 이 밖에 졸피뎀은 cytochrome p450(cyp3a4) 간 효소에 의해 대사되는 약물로 노인이나 간질환 또는...
grabcad
free

불면증개선제~카톡【AKR331】텔레【RDH705】졸피뎀가격,졸피뎀파는곳,졸피뎀사용방법,졸피뎀약효,졸피뎀 처방전
...사용과 주의 깊은 모니터링이 필요하다"고 조언했다. 이 밖에 졸피뎀은 cytochrome p450(cyp3a4) 간 효소에 의해 대사되는 약물로 노인이나 간질환 또는...
grabcad
free

시안화칼륨효과,카톡【AKR331】텔레【RDH705】시안화칼륨약효,시안화칼륨치사량,시안화칼륨파는곳
...우리 몸에 꼭 필요한 중요한 효소 중 하나인 시토크롬 산화효소(cytochrome oxidase)에 포함되어 있는 철 이온(fe3+)과 결합하면서 효소의 기능을 마비시키기...
1A2
turbosquid
$199

Flakpanzer Gepard 1A2
... available on turbo squid, the world's leading provider of digital 3d models for visualization, films, television, and games.
thingiverse
free

Marder 1a2 1:100 TEAM YANKEE by chuso30
...marder 1a2 1:100 team yankee by chuso30
thingiverse
model in 2 pieces with rotable turret. enjoy
thingiverse
free

1:100 Scale Marder 1A2 by kkentium
...es so coming will be the leopard 1, leopard 2a4, leopard 2a5, fuchs, wiesel, and pah1 helicopter. so stay tuned for more designs!
thingiverse
free

Leopard 1 Family by Hushhushrat
...family by hushhushrat thingiverse leopard 1 leopard 1a1 leopard 1a2 leopard 1a3 leopard 1a4 leopard 1a5 flugabwehrpanzer gepard brückenleger...
thingiverse
free

Marder IFV for microarmor by Captain_Ahab_62
...ifv for microarmor by captain_ahab_62 thingiverse marder ivf ( 1a2.. 1a3? no tows though ) modeled in blender 2.79b...
thingiverse
free

Leopard 1 a1 for microarmor
...mbt from west germany this version, the 1a1 and 1a2 both had a cast turret, as opposed to the...
grabcad
free

Double Pitch Chain C2050-1A2
...double pitch chain c2050-1a2
grabcad
double pitch chain c2050 - 1a2
grabcad
free

F-07150F-1A2
...f-07150f-1a2
grabcad
chain flanged roller conveyor
cg_trader
$149

Flakpanzer Gepard 1A2
...s created on real base. it’s created accurately, in real units of measurement, qualitatively and maximally close to the original.
P450
3ddd
$1

Прикроватная тумба
...прикроватная тумба 3ddd прикроватная , тумба текстуры прилагаются l670 p450 ...
3dfindit
free

RT-P450
...rt-p450
3dfind.it
catalog: woodi
thingiverse
free

Cytochrome P450 3A4 by hectorsm
...db id: 1w0e
obtained from:https://www.rcsb.org/structure/1w0e
processed with:
blender (v.2.92.0)
pymol (v.2.4.1)
slic3r (v.1.3.0)
3d_sky
free

Bedside table
...bedside table 3dsky bedside table textures are attached l670 p450 ...
thingiverse
free

Ormerod BLTouch Mount by dapol
...reset the probe in case it has faulted g4 p450 ; wait 450ms for probe to reset m280 p3...
grabcad
free

정품졸피뎀가격,카톡【AKR331】라인【SPR331】졸피뎀구매처,졸피뎀부작용
...주의 깊은 모니터링이 필요하다"고 조언했다. 이 밖에 졸피뎀은 cytochrome p450cyp3a4) 간 효소에 의해 대사되는 약물로 노인이나 간질환 또는 만성...
grabcad
free

불면증개선제~카톡【AKR331】텔레【RDH705】졸피뎀가격,졸피뎀파는곳,졸피뎀사용방법,졸피뎀약효,졸피뎀 처방전
...주의 깊은 모니터링이 필요하다"고 조언했다. 이 밖에 졸피뎀은 cytochrome p450cyp3a4) 간 효소에 의해 대사되는 약물로 노인이나 간질환 또는 만성...
3dwarehouse
free

P450-6 TROPICS
...p450-6 tropics
3dwarehouse
3dwarehouse
free

P450-3 RAINWATER
...p450-3 rainwater
3dwarehouse

