3DWarehouse
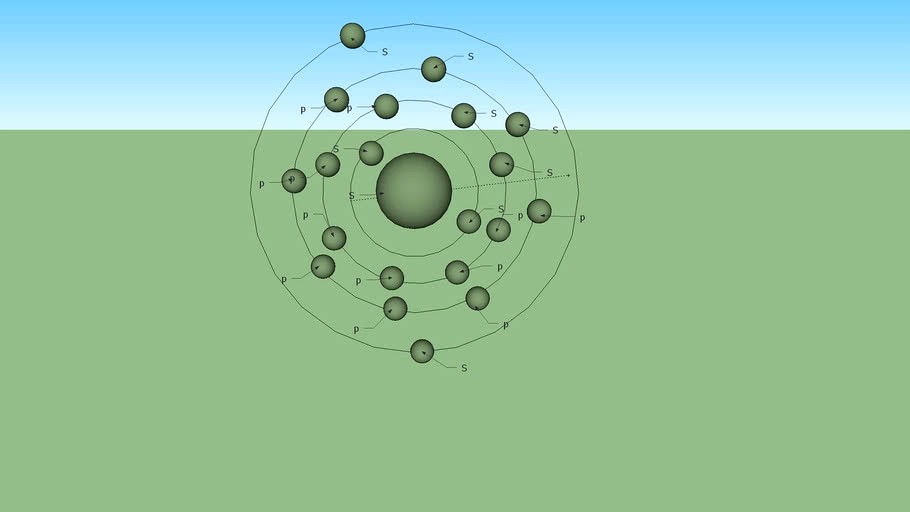
Ca - Calcium
by 3DWarehouse
Last crawled date: 1 year, 10 months ago
Electronic configuration Calcium is the chemical element of atomic number 20. Its symbol is Ca. It is a light earth alkaline metal, gray, used as a reducing agent for mining torsion, uranium and zirconium; When exposed to air, forms a layer of dark oxide. Its physical and chemical properties are similar to its heavier homologues, strontium and barium. It is the fifth most abundant element of the earth's crust and the third most abundant metal, after iron and aluminum. The most common calcium compound found on Earth is calcium carbonate, found in limestone and in fossils dating back to the anesthetic marine life; Plaster, anhydrite, fluorite and apatite are also sources of calcium. The name derives from the Latin calx, 'lime', meaning obtained from the heating of the limestone. Its compounds have been known since antiquity even though their chemistry was unknown until the 17th century. It was first isolated by Humphrey Davy in 1808 by electrolysis of its oxide. While pure metal can not boast many applications due to its high reactivity, it is often used in small quantities as a component of steel alloys, while some lead and calcium alloys are sometimes used in the manufacture of automotive batteries. Calcium compounds are, moreover, widespread in many areas: for example, they are used in the food industry, in the pharmaceutical industry, in the paperboard such as bleach, cement, soap production, and as electrical insulators. Calcium is the fifth most abundant element of the human body and the most abundant metal. Calcium ions play a vital role in physiology and biochemistry of the body and cell as electrolytes. They play an important role in the signal transduction pathways, where they act as a second messenger, in the release of neurotransmitters from neurons, in contraction of all types of muscle cells and in fertilization. Many enzymes require calcium ions as cofactors. External calcium ions also are important in maintaining the potential difference between excitable cell membranes and proper bone formation. #Ca #Ca20 #Calcium
Similar models
thingiverse
free

The Calcium Pump by stemcellgal
...erent configurations: the first one is an open position with no ca ions (pdb id: 1iwo) versus the closed position (pdb id: 1su4).
3dwarehouse
free

Mg - Magnesium
...ements. this alkaline earth metal is mainly used as a binder in the production of aluminum-magnesium alloys. #magnesium #mg #mg12
3dwarehouse
free
W - Tungsten
...t that it is the metal with the highest melting point), but its alloys are also used in the aerospace industry. #w #w74 #tungsten
3dwarehouse
free

Al - Aluminium
...mportant in other transport and construction fields where lightness, durability and durability are required. #al #al13 #aluminium
3dwarehouse
free

Ce - Cerium
...dized to the +4 state. it is the most common of lanthanides, followed by neodymium, lanthanum and praseodymium. #ce #cerium #ce58
3dwarehouse
free

I - Iodine
... thyroid cancer. iodine is also used as a catalyst in the industrial production of acetic acid and some polymers. #i #iodine #i53
3dwarehouse
free

Ac - Actinium
...the series of the actinoids, a group of 15 similar elements of the periodic table between the actinium and...
3dwarehouse
free

V - Vanadium
...ity. particularly in the marine environment, vanadium is used in some forms of life as an active enzyme center. #v #v23 #vanadium
3dwarehouse
free

Si - Silicon
...icon containing compounds, oxygen and metals). silicon is the main component of glass, cement, ceramics and silicon. #si #silicon
3dwarehouse
free

Na - Sodium
...s highly reactive, burns with a yellow flame, oxidizes in contact with the air and reacts violently with water. #na #na11 #sodium
Calcium
thingiverse
free

The Calcium Pump by stemcellgal
...erent configurations: the first one is an open position with no ca ions (pdb id: 1iwo) versus the closed position (pdb id: 1su4).
thingiverse
free

The Calcium Pump by se3dedu
...s against a concentration gradient crossing the membrane. the original file is obtained from the protein data bank (pdb id: 1su4)
thingiverse
free

Naturalistic calcium and food dish by Spazmops
...c feeder dish with an included area for calcium. intended for use with leopard geckos, but could work for other reptiles as well.
thingiverse
free

Salifert Profi Test Calcium storage box by Haggy_23
...ly stowed in this box, after removing the hood, all parts including instructions are easily accessible and nothing can fall over.
thingiverse
free

Red Sea Profi Test Calcium storage box by Haggy_23
...ly stowed in this box, after removing the hood, all parts including instructions are easily accessible and nothing can fall over.
thingiverse
free
nyos test kit holder calcium by proview
...nyos test kit holder calcium by proview
thingiverse
holder for nyos cal test kit
thingiverse
free

Dehumidifier - small for filament box (Calcium Chloride) by Aussie_Jo
... lid screws on tightly. the basket gets filled with calcium chloride (i think the last brand i bought was damprid from bunnings).
thingiverse
free

Aquaforest Calcium test kit - AF CA - Expert Marine by DOGRSBR
...st kit - af ca - expert marine by dogrsbr
thingiverse
base para a organização do kit de teste de cálcio (ca) da aquaforest (af).
thingiverse
free

Lizard Feed Tray with Worm lid and Calcium tray by dx_sniper
...s not cost a million dollars, cue the 3d printer. i printed this with eryon marble and its worked well for him for over 6 months!
thingiverse
free

Calmodulin by aarono
...calmodulin (cam) (an abbreviation for calcium-modulated protein) is a calciumbinding messenger protein expressed in all eukaryotic cells. cam is...
Ca
turbosquid
$10

Clock CA
... available on turbo squid, the world's leading provider of digital 3d models for visualization, films, television, and games.
3ddd
free

Cas Ceramica коллекция Camas
... ceramica cas , восток
ceramica cas восточная коллекция camas, jpeg + coreldraw
turbosquid
$5

ca
...squid
royalty free 3d model car 3d model for download as max on turbosquid: 3d models for games, architecture, videos. (1395842)
turbosquid
free

CA-87 Blaster
...turbosquid
free 3d model ca-87 blaster for download as blend on turbosquid: 3d models for games, architecture, videos. (1177625)
turbosquid
$60

Tatra 148 CAS
...3d model tatra 148 cas for download as 3dm, 3ds, dae, and obj on turbosquid: 3d models for games, architecture, videos. (1642347)
turbosquid
$85

Calgary CA City
...l calgary ca city for download as c4d, 3ds, fbx, obj, and stl on turbosquid: 3d models for games, architecture, videos. (1593925)
3ddd
$1

Обои Zambaiti Parati - коллекция Ca Falzer
...iti parati , ca falzer
коллекция ca falzer фабрики zambaiti parati.
в архиве 55 текстур.
turbosquid
$12

ice tea aluminum ca
... available on turbo squid, the world's leading provider of digital 3d models for visualization, films, television, and games.
3d_export
$10

headphone ca andromeda
...headphone ca andromeda
3dexport
campfire audio model andromeda<br>in the archive left, right earpiece and ambushur
3ddd
$1

CAS SW 5: Весы электронные фасовочные
... фасовка , оборудование
весы электронные фасовочные.
polys: 38392
verts: 35749
